AP Laboratory Solutions
Leading anatomic pathology institutions across academic medical centers, reference laboratories, health systems, and biopharma use AISight®1 as an image management solution.
Image Management System
Powering the next generation of digital pathology labs
AISight is a cloud-native intelligent enterprise workflow solution used by the world's leading laboratories and research centers to power their digital pathology workflows and AI applications. It serves as a central hub for case management, image management, and best-in-class artificial intelligence tools to enable multiple histopathology use cases.
Experience AISight for Yourself

Interoperable
Flexible scanner-agnostic workflow interoperable with most major laboratory information systems (LIS) and scanners (Hamamatsu, Leica, Roche, Philips)

Cloud-based
Browser accessible plus cloud-based image and data storage for rapid activation and scale without the need of additional infrastructure investment
Customizable and Configurable
Flexible workflow to support multiple use cases, with advanced user settings and privileges
Open AI Environment
Integrated PathAI and 3rd-party algorithms to maximize AI deployments across multiple pathologist specialities

Pathologist Centric
Built-in image analysis and collaboration tools for an intuitive and practical user experience

Secure and Private
ISO 27001 certified and follows industry-recognized security and privacy frameworks
LIS Integration
AI digital pathology built around your LIS workflow
AISight Link powers AISight's IMS integration with laboratory information systems (LIS) to augment your existing digital pathology workflow with minimal disruption or additional infrastructure.

100+
Institutions across academic medical centers, reference labs, health systems, and independent pathology labs
25+
AI products accessible for deployment across oncology, MASH, and IBD indications
500K+
Slides analyzed since our launch of AISight
AISight® Multi-Partner AI Portfolio
Seamless Access to AI-Powered Pathology Applications
PathAI’s AISight platform offers an integrated ecosystem of world-class AI algorithms developed by top-tier partners, bringing innovative tools to pathology labs of all sizes and specialties. With the AISight Multi-Partner AI Portfolio, pathologists have seamless access to a diverse array of high-performance digital pathology solutions—all within the AISight Image Management System (IMS).
Curated AI Applications
Access a carefully selected range of AI-powered algorithms from industry-leading developers like DeepBio, DoMore Dx, Paige, and Visiopharm. Each algorithm is tailored to enhance efficiency and support critical tasks, from biomarker quantification to primary diagnosis and prognostication.
Full Integration with AISight IMS
Each application is fully embedded within the AISight IMS, ensuring a smooth, intuitive user experience. This eliminates the need for complex setups, allowing pathologists to access powerful tools directly within the workflows they already know and use.
Scalable, Flexible, and Future-Ready
The AISight portfolio continually grows as new AI applications are added, enabling pathology labs to easily expand their capabilities and stay at the forefront of digital pathology innovation.
A Unified Platform for Enhanced Diagnostic Precision
With deep integrations of third-party algorithms powered by AISight Link, our open API, the AISight Multi-Partner AI Portfolio ensures that labs can leverage advanced features like AI Impressions, Fields of Interest, and detailed reporting tools—all in one cohesive platform designed to elevate diagnostic precision and lab efficiency.

Algorithms for Pathology Assessment
AISight further comes with the industry's largest menu of state-of-the-art and out-of-the-box algorithms1 across research, workflow optimization, assisted scoring, and biomarker quantification, and assistants use cases embedded directly into the IMS.
Quality Control
Automated, instant detection of non-evaluable slides for re-scanning or re-staining
ArtifactDetect2 & Automated Digital Slide QC
- H&E
- IHC
- Trichrome
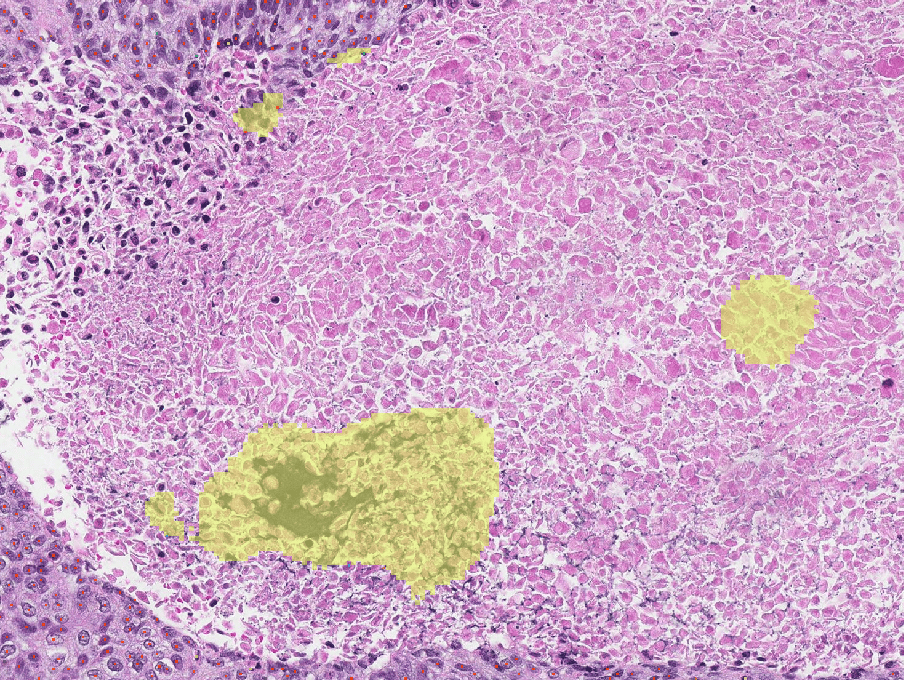
Case Prioritization
Identifying cases with likely tumor can help reduce turnaround time by expediting IHC orders and second reviews
TumorDetect2 for Case Prioritization runs on H&E slides for all solid tumors
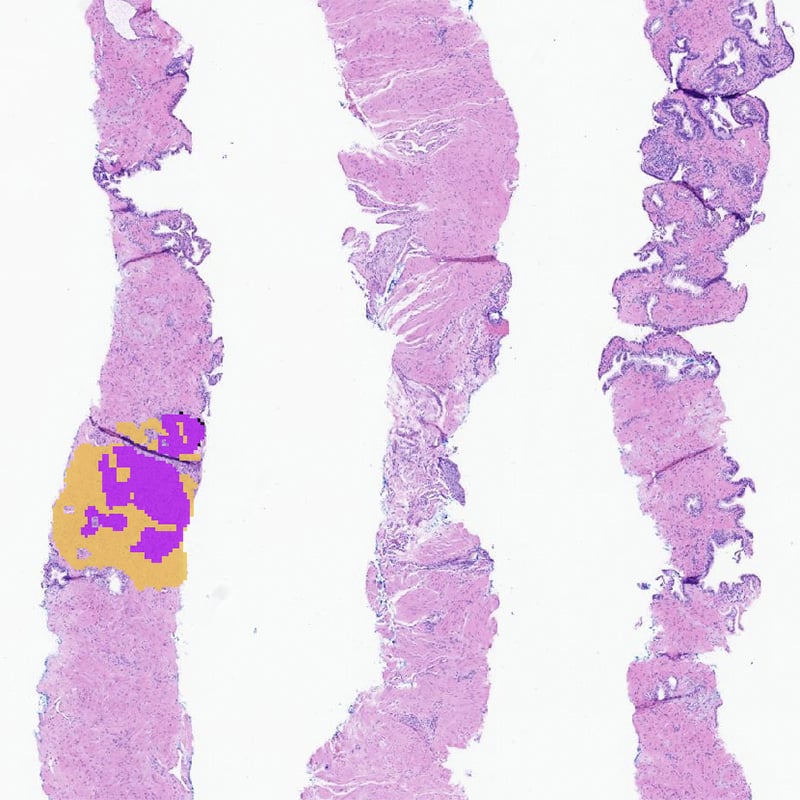
Tumor Cellularity Quantification
Automated, whole-slide level assessment of tumor cellularity, or tumor fraction
AIM-TumorCellularity (AIM-TC2)
Key results:
- Total Tissue Area
- Total Tumor Area
- Percent Tumor Nuclei
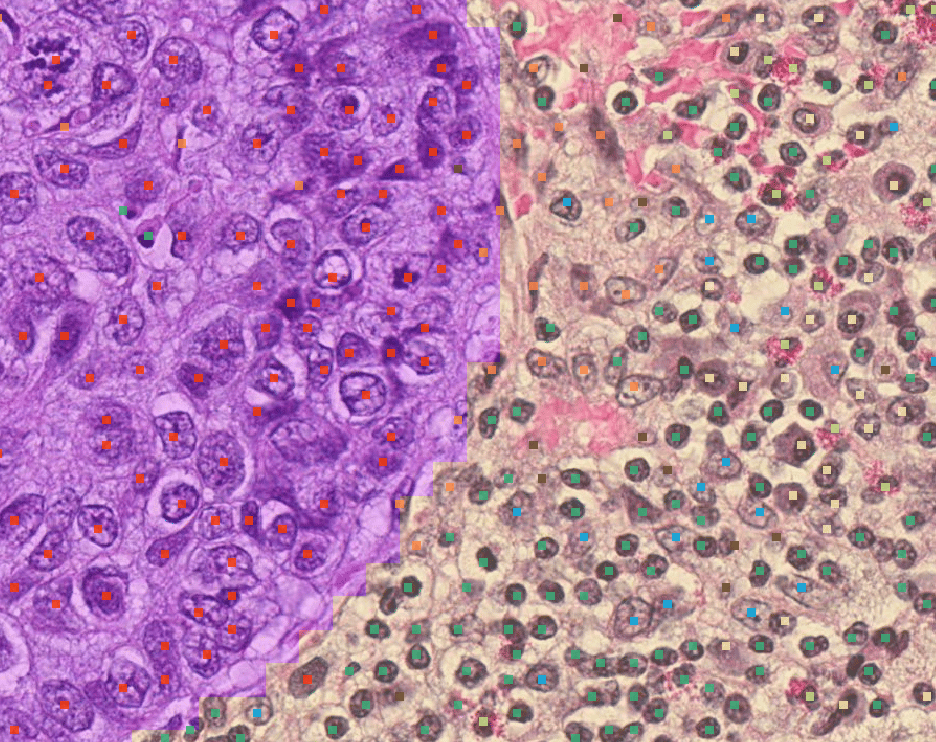
Disease Severity
Accurate and precise assessment and measurement of disease severity
AIM-MASH1
- AIM-MASH+1 (H&E + Trichrome)
AIM-HI UC1
- Ulcerative Colitis (Geboes/Nancy/Robarts H&E)
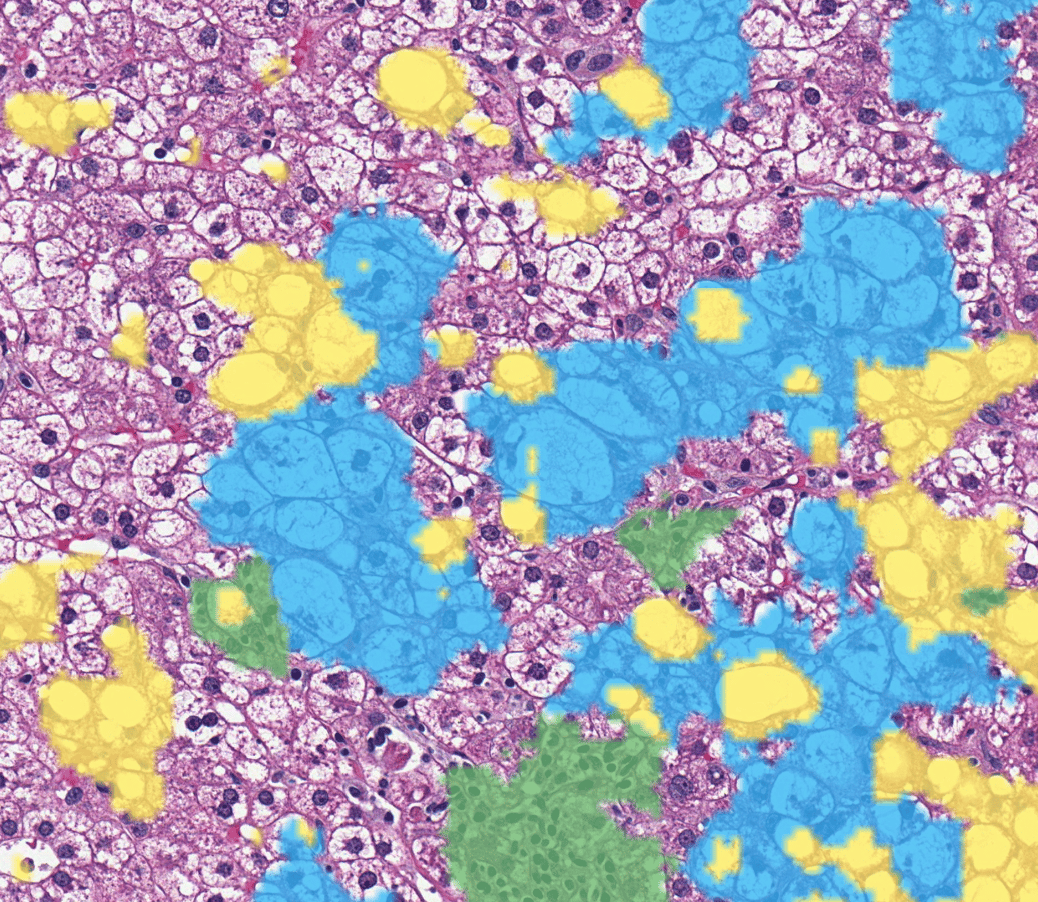
Biomarker Quantification
AI-powered, reproducible biomarker and disease severity assessment
AIM-PD-L11
- NSCLC (Agilent Dako 28-8 and 22C3; Roche Ventana SP142 and SP263)
- HNSCC (Dako 28-8)
- Melanoma (Dako 28-8)
- Urothelial (Dako 28-8)
AIM-HER21
- Breast (4B5 and HercepTestTM)
AIM-ER1
- Breast (Dako EP1, VentanaSP1)
AIM-PR1
- Breast (Dako PgR 636, Ventana 1E2)
AIM-Ki-671
- Breast (Dako MIB-1, Ventana 30-9)
.webp?width=1924&height=1226&name=Screenshot-2023-07-27-at-2%20(1).webp)
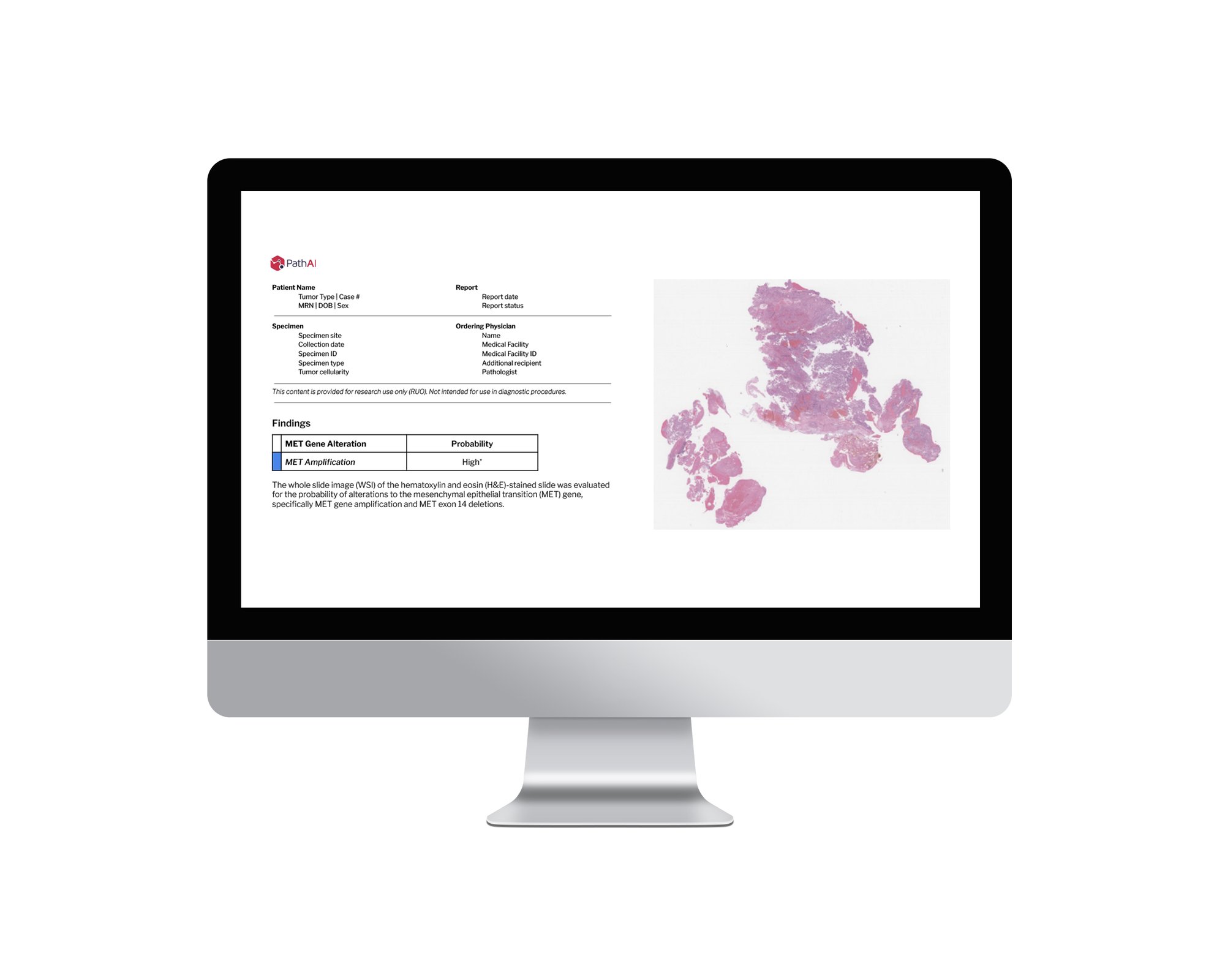
Molecular Prediction Algorithms
Biomarker identification from H&E WSI to accelerate and expand clinical trial enrollment
- MET Dysregulation (NSCLC)1
AISight Featured Resources
.webp)
Connect with our team
To learn more about how AISight1 and our menu of algorithms can be used in your lab, contact us at digitaldx@pathai.com.
1AISight® and PathAI Algorithms (including AIM-PD-L1, AIM-HER2, AIM-ER, AIM-PR, AIM-Ki-67, AIM-MASH+, MET Dysregulation NSCLC, AIM-HI, and PathAssist Derm are For Research Use Only in the United States. Not for use in diagnostic procedures.
2ArtifactDetect, CasePriority, and AIM-TumorCellularity are workflow tools accessed via the AISight Image Management digital pathology platform. AISight® is for Research Use Only in the United States. AISight® Dx is FDA-cleared for primary diagnosis in the US with the Hamamatsu NanoZoomer® S360MD, Leica Aperio® GT 450 DX, Roche VENTANA® DP200 and DP600 slide scanners and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland.

