We Bring Powerful New Technologies to Pathology that can Improve Patient Outcomes
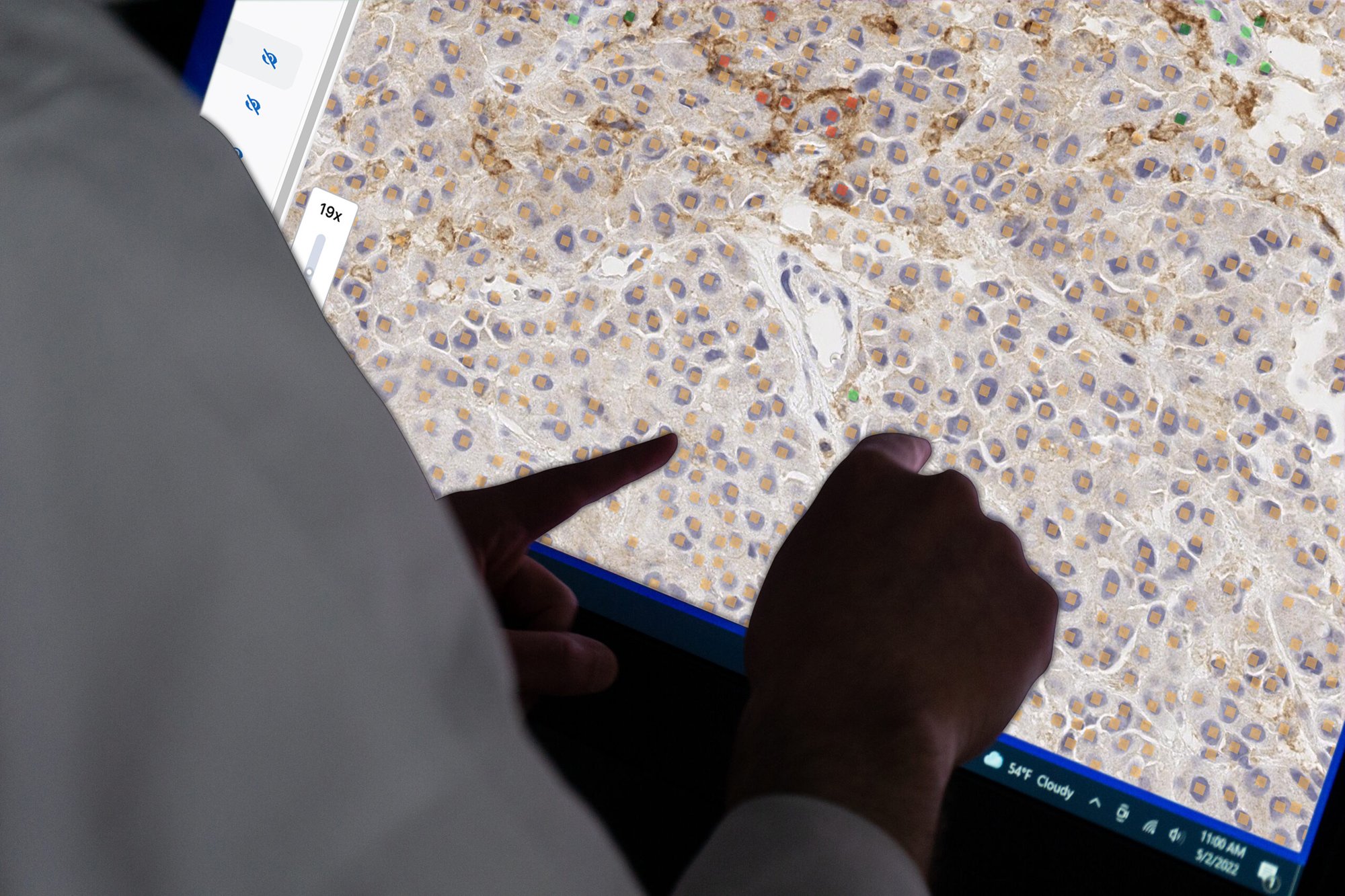
We are transforming pathology by developing AI models that optimize the analysis of patient tissue samples and support our customers with solutions from wet lab services to algorithm deployment for clinical trials and diagnostic use.
The only AI-focused technology company with comprehensive precision pathology solutions
We develop robust, versatile precision pathology solutions with our deep computational and medical expertise.
We leverage data from more than 32.5 million annotations provided by our proprietary and crowd-sourced pathology network of over 450 pathologists, and a vast library of archived data to rigorously train and validate our AI-based models.
This allows us to deliver quality and transformative tools for pathologists that can help accelerate drug development, improve confidence in the accuracy of diagnosis, and get life-saving therapies to patients more quickly.
Our mission at PathAI is to improve patient outcomes with AI-powered pathology through:

More Accurate Diagnosis

Matching Patients with the Most Effective Treatments

More Life-Saving Drug Approvals
Leadership Team
Our leadership team combines industry-leading expertise across diverse fields – engineering, machine learning, life sciences, medicine, regulatory, strategy, and operations – to help drive the PathAI mission forward with everyone in our organization.

PathAI has established itself as a leader in AI-powered precision pathology through significant moments that accelerated growth
2016 - Founded
Founded in 2016, our Co-Founders identified the need for technology applied to pathology to reduce error rates, increase accuracy and reproducibility.
2019 - Received ISO 13485 and ISO 27001 Certifications
PathAI received certification of its quality management system for compliance with ISO 13485 and MDSAP for US regulations that validates its approach to produce safe and effective medical device software to support pathologists. PathAI also received its ISO 27001 certification, one of the highest internationally-recognized standards for information security management systems.
2019 - First Abstract Published in Journal of Clinical Oncology
PathAI's first published abstract titled CD8+ T cells in tumor parenchyma and stroma by image analysis (IA) and gene expression profiling (GEP): Potential biomarkers for immuno-oncology (I-O) therapy, is released for the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting.
2020 - First Patents Issued
PathAI is issued its first two patents, which teach predicting a survival time of a patient and predicting tissue characteristics for a pathology image.
2020 - First Manuscript Published in Hepatology
PathAI's first manuscript titled Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH, is published in Hepatology, Journal of the American Association for the Study of Liver Diseases (AASLD).
2021 - Secured $165M Series C Funding
PathAI raised $165 million in Series C funding. This round fueled PathAI’s commercial reach in our research and development capabilities.
2021 - Expanded into Clinical Diagnostics
PathAI entered clinical diagnostics through the acquisition of Poplar Healthcare, an industry-leading independent anatomic pathology laboratory services provider.
2022 - In-house BioPharma Lab Built
PathAI builds in-house CAP/CLIA-certified GCLP histopathology lab to fully support end-to-end clinical trials for its BioPharma partners.
2022 - Received FDA 510(k) Clearance for AISight® Dx
PathAI received FDA 510(k) clearance and CE Mark for its digital pathology platform, AISight Dx, which can be used for primary diagnosis in clinical settings.
2023 - Launched over 20 cutting edge AI products
PathAI launched over 20 cutting edge AI products, including PathExploreTM, PathAI's flagship explore product, which provides structured, standardized, and scalable panel of human interpretable features (HIFs) and delivers unparalleled insight into the tumor microenvironment (TME) from H&E whole-slide images.
2024 - Announced Strategic Partnership with Quest Diagnostics
Partnership with Quest Diagnostics included sale of PathAI Diagnostics and licensing of AISight platform.
2025 - Launched Precision Pathology Network
PathAI launched the Precision Pathology Network(PPN), the first global network of digital anatomic pathology laboratories.
2025 - AISight® Dx Received FDA Clearance
PathAI received FDA clearance for the evolved AISight® Dx digital pathology platform for primary diagnosis, including a Predetermined Change Control Plan (PCCP) to enable specific future enhancements without new submissions.
2025 - AIM-MASH AI Assist Expanded Regulatory Qualification
PathAI’s AIM-MASH AI Assist received qualification from the European Medicines Agency (EMA) and became the first AI-based tool to receive FDA qualification for MASH clinical trials.

Get In Touch
Interested in learning more about PathAI and how we're transforming pathology?

.png)




