Navigate the future of digital pathology with
AISight Dx Digital Pathology Platform
(CE-IVDR Certified)
.png?width=2000&height=2000&name=Landing%20page%20header%20use%20graphic%20(1).png)
Introducing AISight Dx
Powering the next generation of digital pathology labs.
AISight Dx is a CE-marked, IVDR-certified digital pathology platform and Image Management System for primary diagnosis, centralizing case and image management in an AI- and cloud-native solution. It enables pathologists to view, interpret, share, and manage whole slide images intuitively.

Optimize Operational Efficiency
AISight Dx automates workflows, prioritizes urgent cases, and streamlines tasks, reducing manual effort and enabling efficient case management with a modern, everyday-use interface.
Enhance Collaboration
AISight Dx enables interdisciplinary communication and teamwork with both real-time consultation tools such as AISight Live and many asynchronous collaboration tools.
AI-Native
AISight Dx was intentionally designed to integrate AI in the slide viewer and beyond to enable next-generation efficiency through case prioritization, automated assignment, assisted reporting, and quality assurance.
Versatile Functionality
AISight Dx supports a wide range of use cases including diagnostics, research, education, and training to streamline workflows and reduce the need for multiple systems.
End-to-End Pathology Workflow Support
AISight Dx goes beyond standard IMS functionalities and increases efficiency though modern, AI-powered featured through the end-to-end workflow. AISight Dx is the single digital workplace for the modern pathologist.
Pre-Analytic
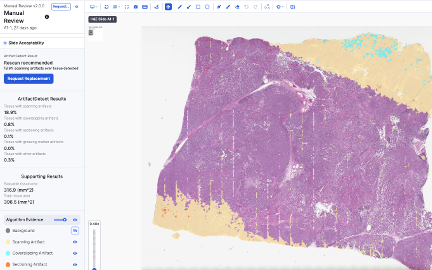
Automated Slide Quality Control
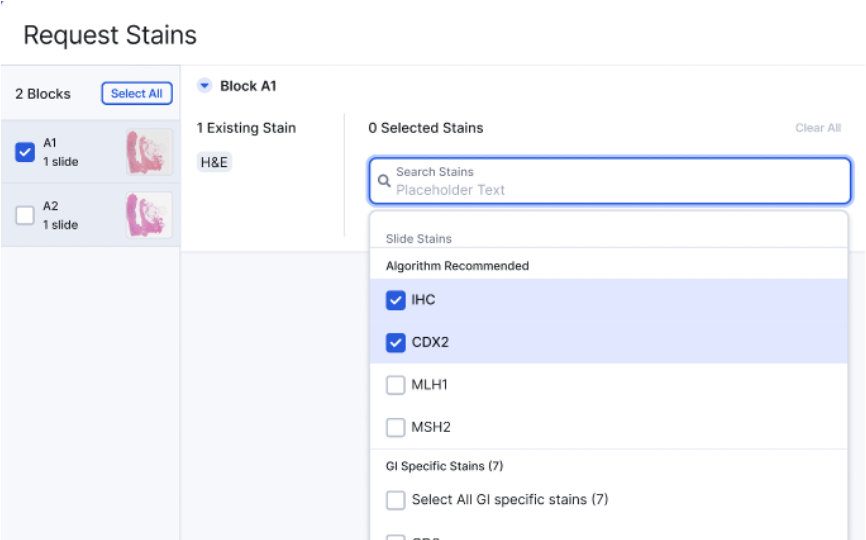
Auto & Manual Lab Orders
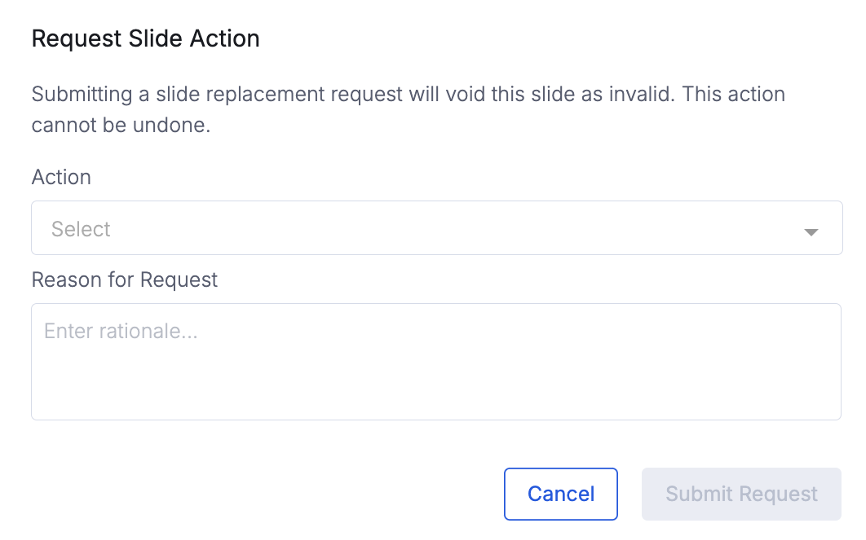
Slide Replacement
Prioritization & Assignment
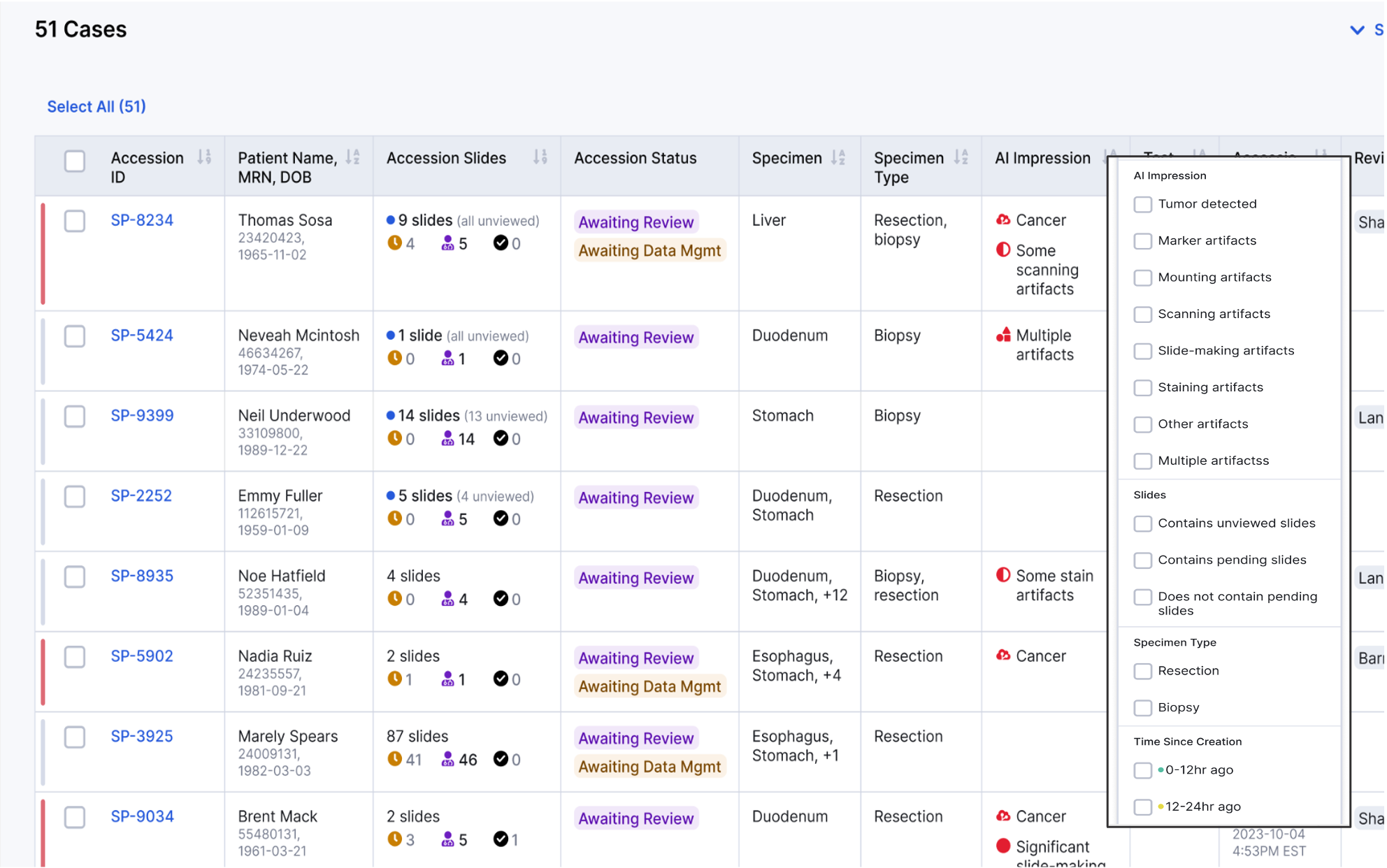
Case Prioritization
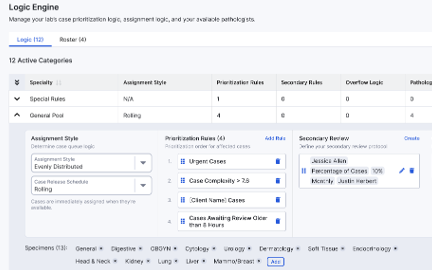
Intelligent Case Assignment
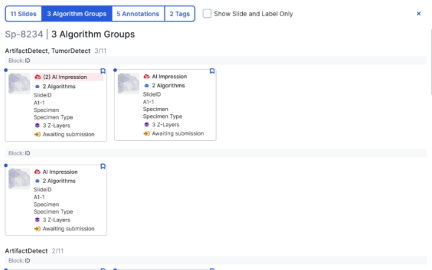
Case Summary
Automation in Case Review*
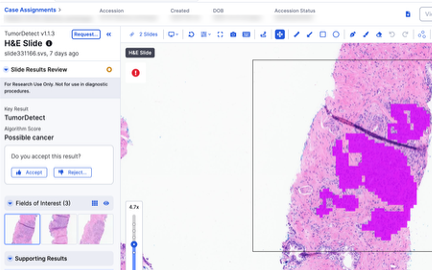
Guided Review
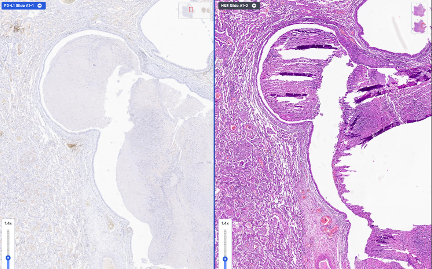
Automated Multi-Slide Alignment
Smart Rotation
Collaboration

Live & Deferred Collaboration

User Tagging & Notifications
Tumor Board Administration
(Assisted) Diagnosis*
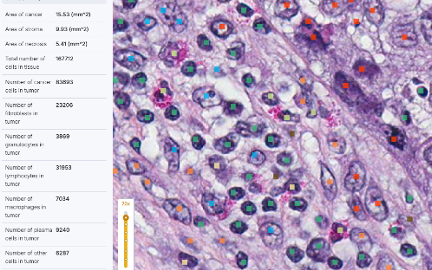
Cell-Level Quantification
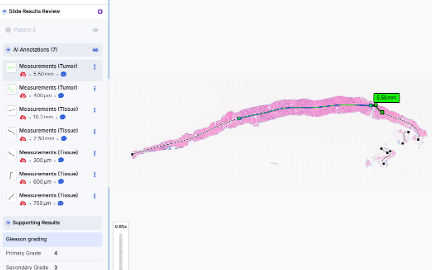
Automated Measurements
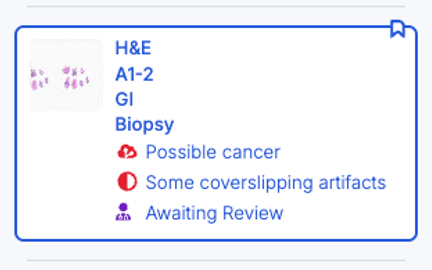
Case- and Slide-Level Synthesis
Reporting & Finalization*
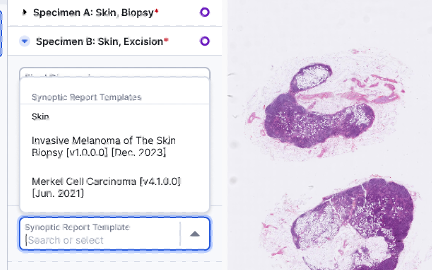
Structured Reporting
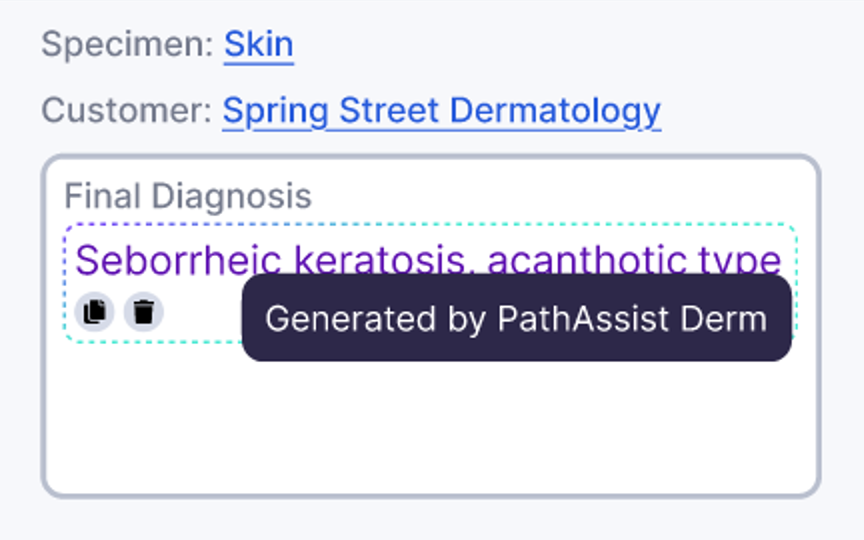
Automated Report Pre-Filling
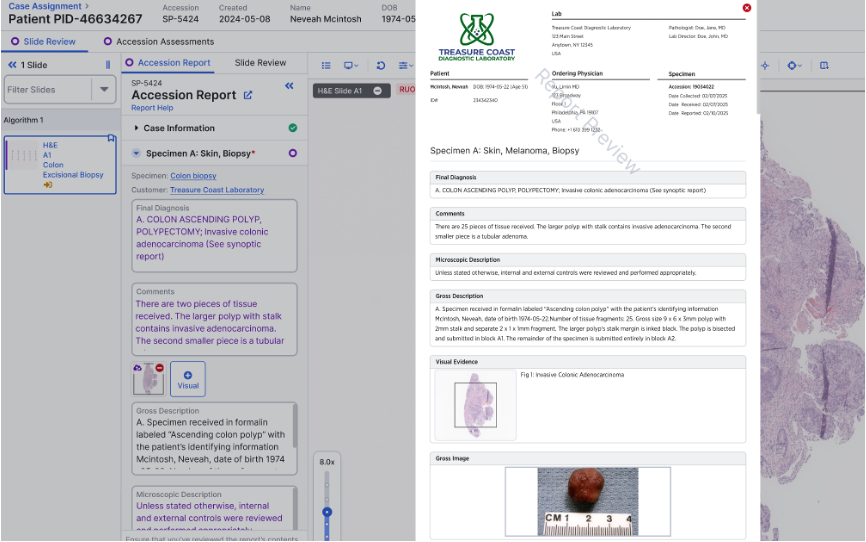
Report Generation
Monitoring
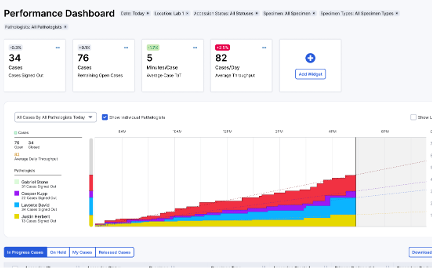
Progress Dashboard
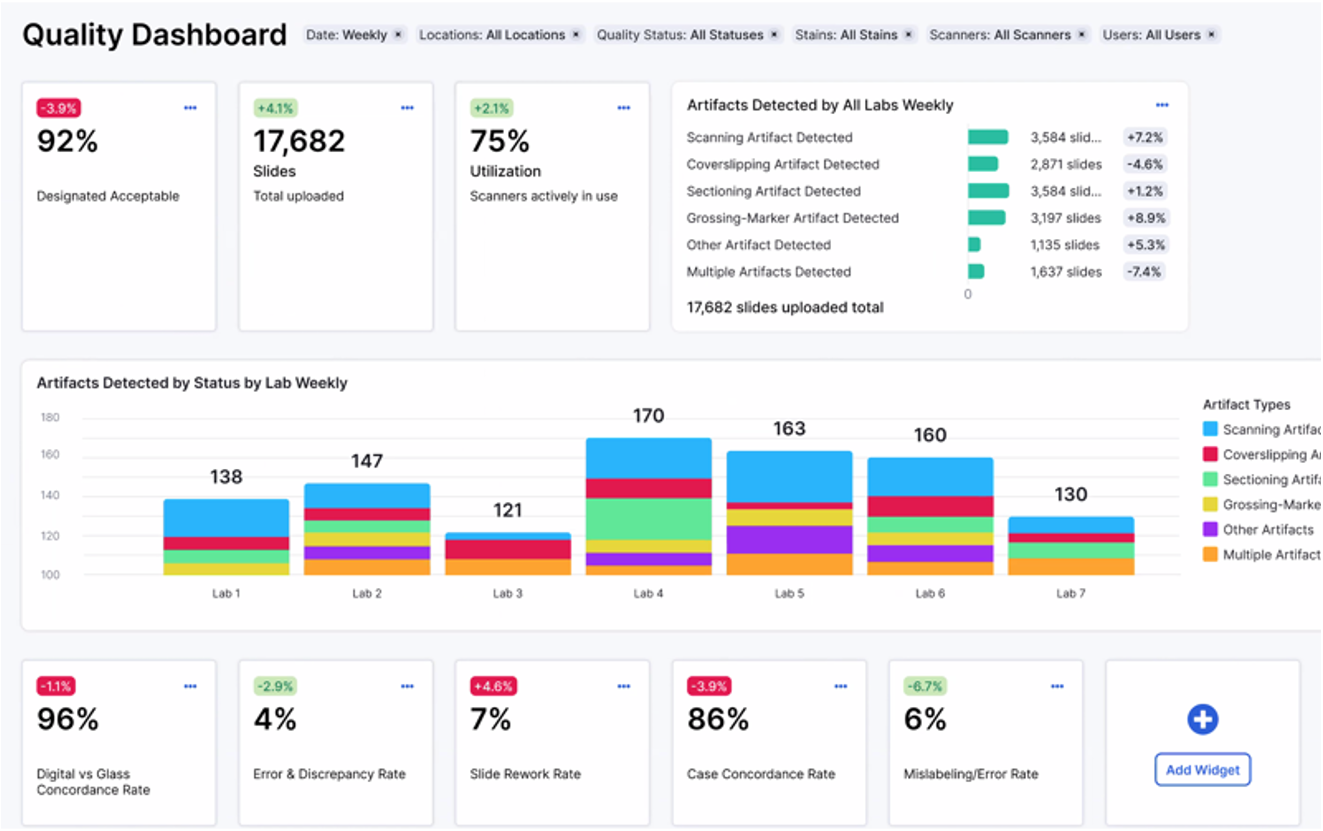
Quality Monitoring
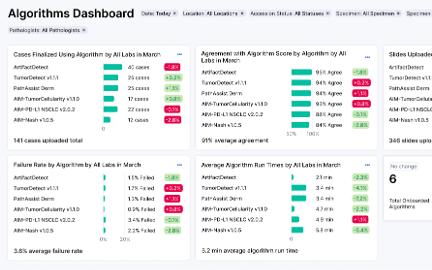
Algorithm Management
Some features are currently in development and available to select partners only, and will be released to production shortly.
*Automation in Case Review, Assisted Diagnosis, Reporting and Finalization features are RUO-only and are not for primary diagnostic use.
Your Partner for a Successful AI Adoption Journey
As a leading IMS provider and AI developer, PathAI combines deep algorithm-development expertise with real-world experience implementing AI in our own routine pathology lab.
AI EXPERTISE & GUIDANCE
As recognized leader in AI development, PathAI supports labs in adopting and integrating AI through expert guidance and optimization, well beyond simply providing algorithms.
CURATED AI APPLICATIONS
Access the largest suite of AI-powered tools, designed by leading digital pathology experts, to deliver high performance and accuracy for evolving lab needs.
SEAMLESS INTEGRATION
All algorithms are fully embedded to create a smooth and intuitive user experience. No need for additional viewers to enjoy the full capabilities of the applications.
An open AI ecosystem in a single platform
PathAI's Workflow Algorithms
The CE-IVDR-certified AISight Dx Image Management System comes with AI-powered workflow functionalities for quality control, case prioritization and tumor sufficiency assessment.

ArtifactDetect
Detects artifact by action-oriented classes (scanning, cover slipping, sectioning, grossing and other) to monitor quality.
.png?width=685&height=561&name=Untitled%20(685%20x%20561%20px).png)
Case Priority
Prioritizes cases based on whether suspicious tissue is identified. Also available as the RUO algorithm TumorDetect, which differentiates tumor, stroma, and necrosis tissue.
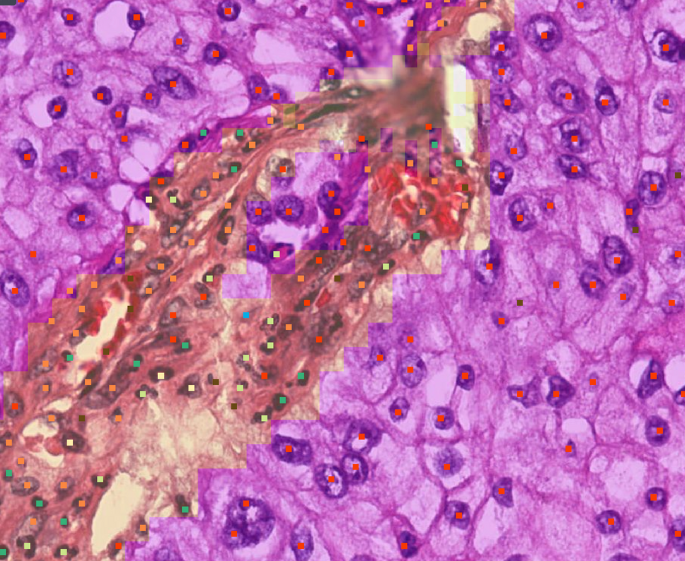
AIM-Tumor Cellularity
Assesses tumor cellularity and tumor nuclei count for pre-analytical workflows before next-generation sequencing.
The Single Platform for All AI Deployments
AISight Dx supports a growing list of 3rd party algorithms (currently 40+) from the world’s leading AI developers for use in research, workflow optimization, assisted scoring, and biomarker quantification. They are fully embedded within the same viewer and across the entire AISight Dx-supported workflow.

PathAI offers a broad RUO AI portfolio, incl. PathAssist Derm for dermatopathology, and biomarker panels such as AIM-PD-L1, AIM-MASH, and AIM-Breast, as well as specialized exploratory research tools.
DeepBio’s DeepDx Prostate is a CE-IVD-marked diagnostic aid designed to enhance the precision of prostate cancer assessment in digital pathology. With DeepDx Breast, DeepBio also offers RUO breast solutions.
Paige’s AI applications alleviate time and resource pressures for pathologists. Paige’s AI suite includes solutions for Prostate, Breast, Colon, and Pan-Cancer.

Primaa offers AI-powered diagnostic tools such as Cleo Breast (CE-IVDR) and Cleo Skin (RUO) designed to detect and quantify cancer biomarkers, lesions, dimensions, margins, mitoses, and perineural invasion.

Histotype Px® Colorectal is an outcome prediction marker for stage II and III colorectal adenocarcinoma that informs the decision of whether to provide adjuvant chemotherapy following surgical resection. It is CE-IVD-marked in EU.

Mindpeak delivers CE-IVD-marked AI solutions for breast cancer biomarker analysis (HER2, ER/PR, Ki-67) and lung PD-L1, complemented by RUO assays for prostate, gastric, esophageal, urothelial cancers, and dermatopathology.

Stratipath provides the first CE-IVD marked AI-based solution for prognostic risk profiling of breast cancer using routine histopathology slides.

Visiopharm offers a full breast panel of APPs (ER, PR, Ki-67, Metastasis Detection, and HER2) and an APP for non-small cell lung cancer (PD-L1). These APPs are cleared under IVDR in EU and UK and RUO in the rest of world.
Trusted Worldwide
AISight Dx is trusted by some of the world’s leading pathology laboratories and life science organizations to support high-throughput, AI-enabled research. The platform processes over 100 million whole-slide images, enabling scalable, efficient, and accurate digital pathology workflows that accelerate discovery and innovation across global institutions.
Explore how AISight Dx is transforming pathology through global partnerships, access the latest updates in our Newsroom.
PathAI and University Hospital Zurich Announce Collaboration to Deploy AISIght® Dx and AIM-Tumor Cellularity for Routine Molecular Pathology Workflows
Leading Austrian Pathology Laboratory Group Chooses PathAI’s AISight® Dx to Accelerate Digital Pathology and Advanced Diagnostics
PathAI's AISight Dx Image Management System is CE Marked for Primary Diagnosis
CASE STUDY
AI-Assisted Titer Selection in Early Assay Development
- PathAI deployed IHC Explore on prostate cancer specimens stained with a novel, in-development assay
- IHC Explore quantifies staining intensity at single-cell resolution, enabling rapid assay characterization and titer optimization
- Continuous staining intensity measurement provides added value for next-generation biomarkers and precision medicine strategies
Schedule a free demo today
fuel your pathology workflows & research with AISight Dx
Diese Seite auf Deutsch besuchen | Voir cette page en français
AISight® Dx is CE-IVD-marked for primary diagnosis in the EEA, CH, and UK. The CE-IVD-marked version of AISight Dx is distinct from, and not identical to, the FDA-cleared version available in the United States.
ArtifactDetect and CasePriority are AISight Dx workflow tools. AIM-TumorCellularity is an AISight Dx workflow tool and not intended to be used for diagnostic purposes in the EU/UK.
TumorDetect, AIM-MASH, AIM-HI Ulcerative-Colitis, AIM-PD-L1, AIM-IHC Breast Panel (HER2, ER, PR, Ki-67) and PathAssist Derm are for research use only. Not for use in diagnostic procedures.

.png?width=1024&height=1024&name=Hubspot%20use%20hexagon%20image%20(1).png)
.png?width=1024&height=1024&name=Hubspot%20use%20hexagon%20image%20(2).png)
.png?width=1024&height=1024&name=Hubspot%20use%20hexagon%20image%20(3).png)


