Leveraging Real-World Data for Real-World Impact
Integrate quantitative and structured pathology data with clinico-genomic real-world data sets to:
- Discover novel histopathology biomarkers
- Enhance clinical development and trial design
- Assess real-world prevalence and treatment effectiveness
.jpg)
What is real-world data and how is it leveraged for drug development?
What is RWD?
Clinical Trial Operations: Enhancing clinical trial protocol development, study feasibility, and population-level eligibility assessment
Regulatory Decision-Making: Supporting new drug approvals or post-approval requirements through continuous data collection and surveillance
Health Economics and Outcomes Research: Generate evidence and inform market access decisions by analyzing real-world clinical and cost-effectiveness
What does RWD contain?
Real-world data is collected through a variety of sources, but generally contains information that captures the complete patient journey from disease diagnosis, biomarker testing, treatment selection, and outcomes. Examples of RWD sources include:
- Electronic Health Records (EHRs)
- Claims and Billing Data
- Patient Registries
- Wearable Devices and Health Apps
- Patient-Reported Outcomes (PROs)
- Molecular Biomarker Tests
What are the benefits of RWD?
Patient Diversity: Captures data from diverse and different patient populations, including those not typically included in clinical trials
Speed and Cost-Effectiveness: Using readily available data helps reduce the time and cost associated with clinical trial operations and data collection
Real-World Insights: Provides a continuous, longitudinal view into patient journeys, treatment patterns, adverse events, and outcomes
Utilizing RWD for drug development
Clinical Trial Operations: Enhancing clinical trial protocol development, study feasibility, and population-level eligibility assessment
Regulatory Decision-Making: Supporting new drug approvals or post-approval requirements through continuous data collection and surveillance
Health Economics and Outcomes Research: Generate evidence and inform market access decisions by analyzing real-world clinical and cost-effectiveness
RWD has driven significant progress in drug development, but pathology data remains underutilized due to a lack of standardization, structure, and scale
Pathology is the critical data element bridging the molecular drivers of disease to their clinical manifestations
Pathology is the only data modality to:
- Define the patho-physiology of disease
- Capture complex disease manifestations underpinned by molecular alterations
- Quantify morphological changes resulting from treatment or disease progression
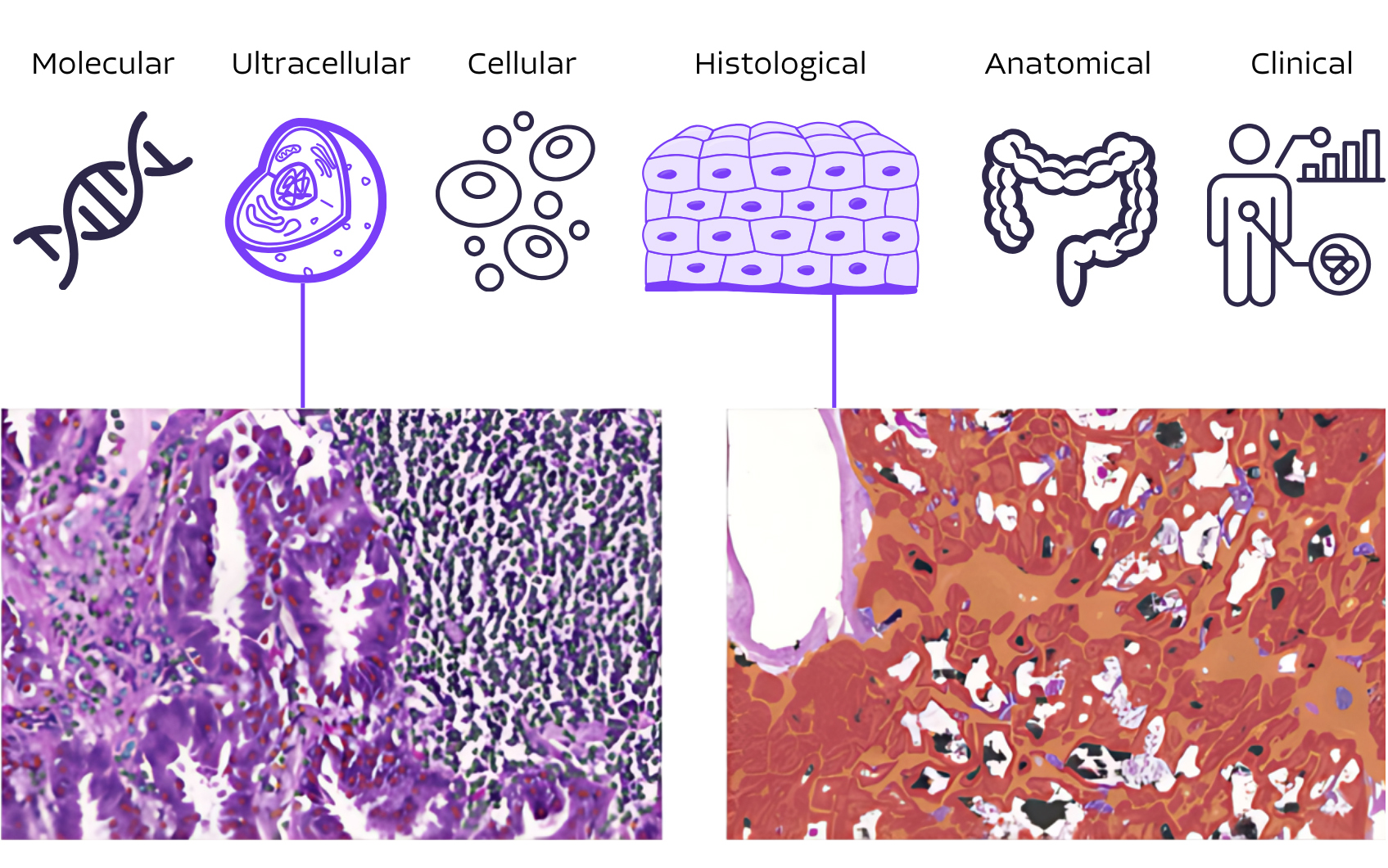
PathAI transforms unstructured pathology images into structured pathology insights to enable seamless integration with clinico-genomics RWD
- Advances in digital pathology and machine learning are now enabling researchers to use routine histopathology samples to obtain a single-cell, spatially-resolved view of tissue structures and cell populations.
- PathAI’s products transform unstructured digital images into human interpretable features (HIFs) allowing for more detailed and actionable insights from histopathology data.
- Integrate quantitative pathology data with other RWD to understand disease intricacies at molecular, cellular, histological, and anatomical levels
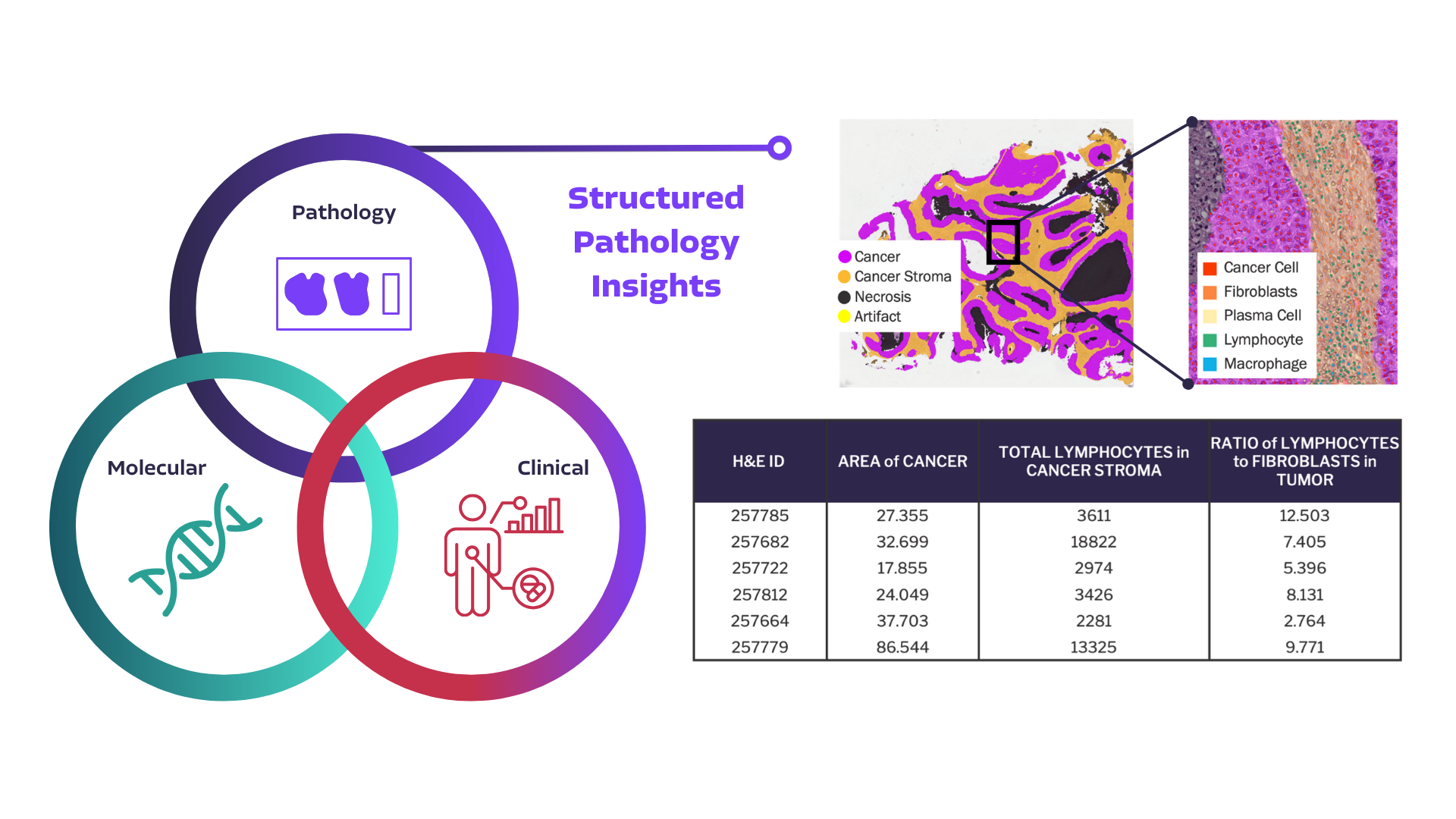
Power your RWD with AI-powered Pathology
See what you can extract with PathExplore™ for quantitative histopathology
PathExplore delivers >300 histopathology features including:
- Total Area of Tumor Tissue
- Total Number of Lymphocytes
- Density of Lymphocytes in Cancer Stroma
- Number of Lymphocytes in Proximity to Cancer Cells
- Spatial Distribution of Tumor Infiltrating Lymphocytes and more
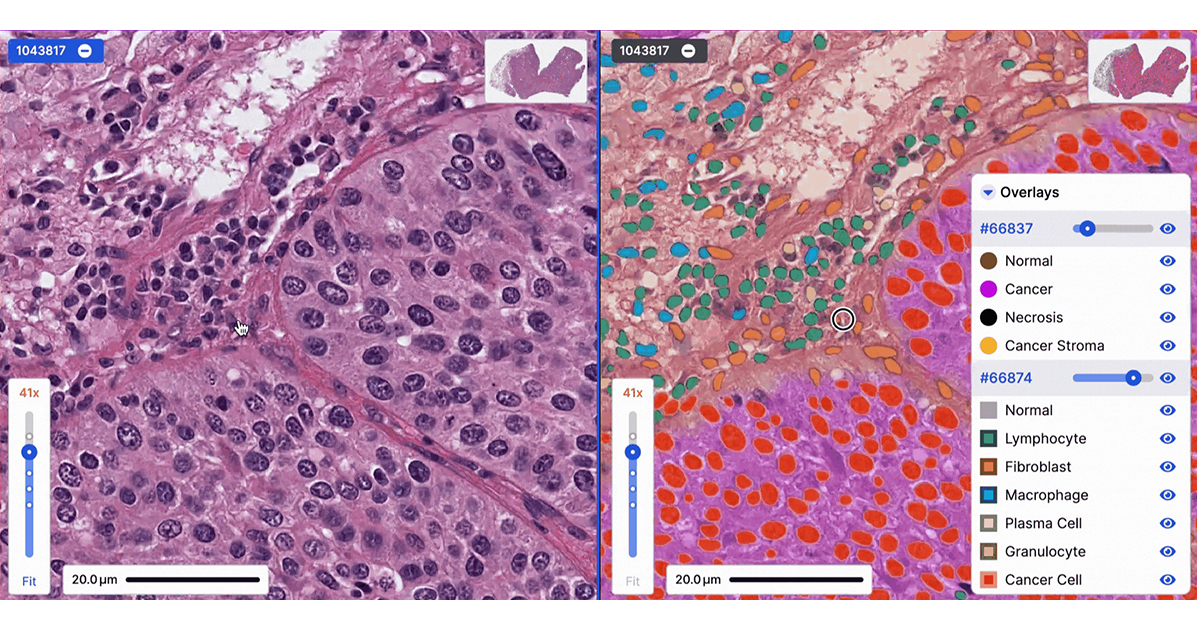
How to work with PathAI: Enhance your drug development with AI-powered pathology
Looking for a real-world dataset?
PathAI has built a network of industry-leading RWD partners, including Aster Insights and ConcertAI, allowing you to access a comprehensive range of curated real-world datasets. These datasets include clinical, molecular, and AI-derived quantitative pathology data, enabling multi-modal analyses across the drug development lifecycle. These datasets are available across oncology indications, including lung, breast, colorectal, melanoma, gastric, kidney, pancreatic, prostate, lymphoma, ovarian, bladder, head & neck, and liver cancers to address your specific use cases.
PathAI also licenses the PathExplore data generated from H&E WSIs from The Cancer Genome Atlas (TCGA), allowing researchers to analyze quantitative pathology features alongside clinical and molecular data from TCGA.
Available datasets
PathAI offers academic researchers access to PathExplore's HIFs associated with publicly available H&E WSI from The Cancer Genome Atlas (TCGA). This program allows researchers to access PathExplore HIFs via our digital pathology platform AISight® to use alongside existing clinical and molecular data from TCGA.
This data is free to academic researchers, and available via license for non-academic partners. To learn more and apply to the PathExplore data access program, please contact pathexplore.hifs@pathai.com. Non-academic researchers may additionally reach out to bd@pathai.com.
Looking to enrich an existing RWD cohort?
Reach out to us to enrich your RWD with AI-powered pathology. Our solutions transform raw WSIs into standardized and structured tissue and cell insights, enhancing your ability to derive meaningful insights and accelerate discoveries in translational research and clinical development.
PathAI Real-World Data Resources