Central Pathology Lab Operations
State-of-the-art central lab powering clinical trials with AI digital pathology
Artificial intelligence will transform the next generation of precision medicine clinical trials
Unlock PathAI's cutting-edge biomarker and scoring algorithms for endpoint analysis and patient enrollment with our central pathology lab that seamlessly integrates AI into clinical trial workflows. Our specialties include NASH, IBD and Oncology.
Introduction to PathAI's Biopharma Lab Services
Introduction to PathAI's Biopharma Lab Services
Introduction to PathAI's Biopharma Lab Services
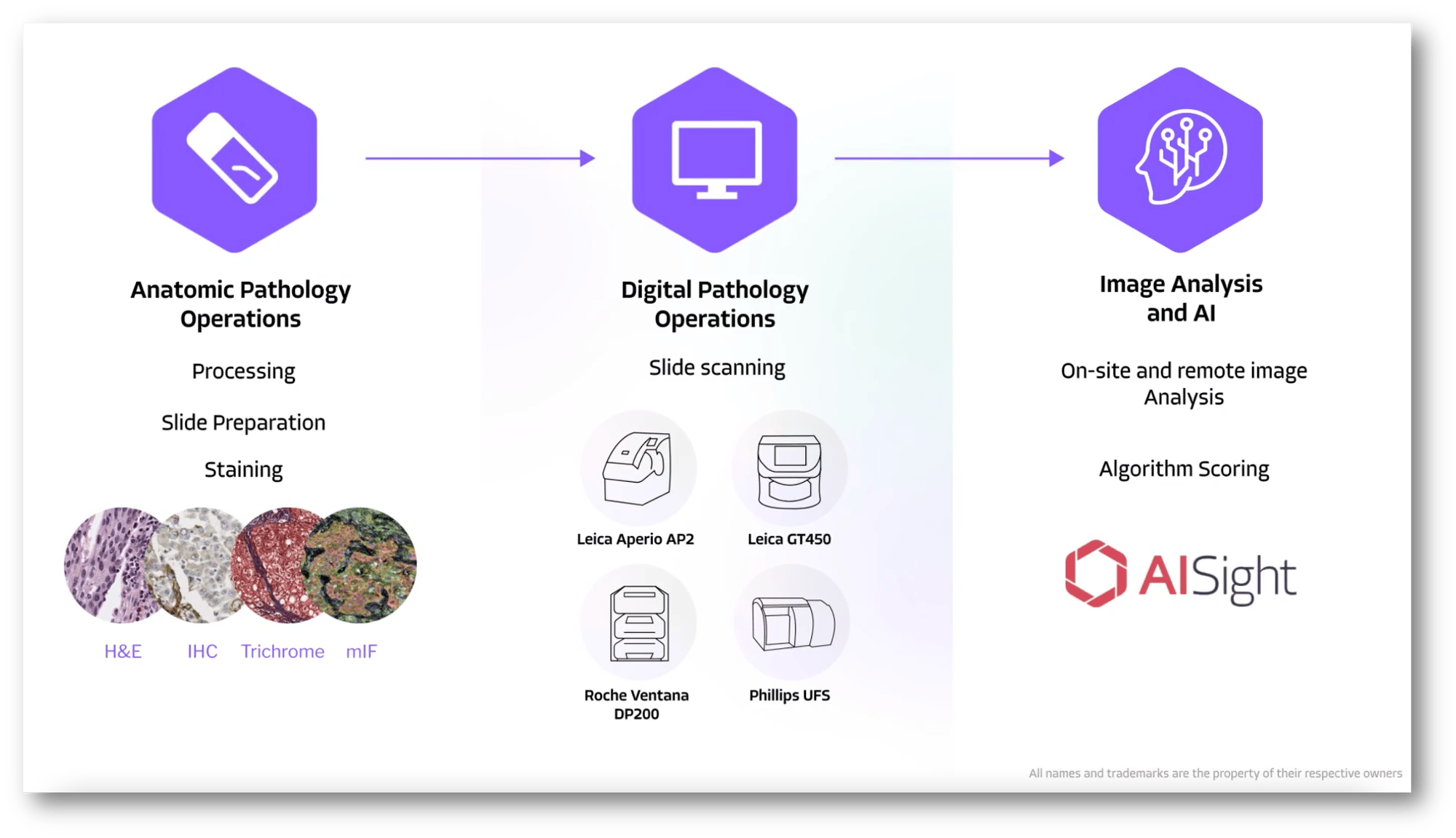
End-to-End Pathology Operations & Services

Pathology Read Strategy
Clinical Development
Site Training
.webp?width=100&height=100&name=Slide-Prep-15%20(1).webp)
Receipt, Processing & Staining
Scanning & Digitization

Manual Central Pathology Reads
Image Analysis
AI-powered Reads

Reporting
Query Resolution
Data Transfer
Establishing the highest level of trust with our partners
- 8 successful audits by biopharma and CROs
- ZERO critical findings in audits to date
- 550+ board-certified pathologists available for central reads
- 2:1 low trial manager-to-trial ratio for high touch service and secure data delivery
- 12 active or upcoming trials across >8 biopharma partners

Our focus and deep expertise matters when trial success critically depends on pathology strategy and operations

Unmatched operational excellence
Our focus and deep expertise in pathology maximizes quality and compliance without compromising turn-around time
.webp?width=100&height=100&name=Check-List-13%20(1).webp)
Comprehensive and fit-for-purpose
Full end-to-end anatomical and digital pathology operations, from block receipt through slide digitization, with multiple staining equipment & scanners for flexible protocols

White-glove partnership
Dedicated clinical trial and data managers to shape the optimal pathology read strategy, ensure robust site training, organize operations across multiple sites and partners, and rapidly resolve challenges
Our Central Pathology Lab Services
AISight Clinical Trials
We power clinical trials with our compliant AISightTM digital pathology platform for algorithm deployment and image analysis, specially designed for clinical trial workflows.


Contact Us
Connect with our team to learn more about PathAI's state-of-the-art central lab and how it's powering clinical trials with AI
1AISight® and PathAI Algorithms (including AIM-PD-L1, AIM-HER2, AIM-ER, AIM-PR, AIM-Ki-67, AIM-MASH+, MET Dysregulation NSCLC, AIM-HI, and PathAssist Derm are For Research Use Only in the United States. Not for use in diagnostic procedures. AISight® Dx is FDA-cleared for primary diagnosis in the US and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland.
2ArtifactDetect, TumorDetect, and AIM-TumorCellularity are workflow tools accessed via the AISight Image Management digital pathology platform. AISight® is for Research Use Only in the United States. AISight® Dx is FDA-cleared for primary diagnosis in the US with the Hamamatsu NanoZoomer® S360MD and Leica Aperio® GT 450 DX slide scanners, and is CE‑IVD–marked for primary diagnosis in the EEA, UK, and Switzerland.